
Current Perspectives in Angle Closure Management and the Evolving Role of Laser Peripheral Iridotomy
By THOMAS SIEMPIS, MD, FRCOphth, and MATT SCHLENKER, MD, FRCSC
Angle closure is an aggressive and blinding disease. We have now developed a deeper understanding of its pathophysiology due to the use of imaging tools such as anterior segment optical coherence tomography and ultrasound biomicroscopy. Recent landmark studies such as ZAP and EAGLE have changed our management approach, with a shift from the universal use of laser peripheral iridotomy in patients with angle closure to earlier lens-based surgery. Combined phacoemulsification with filtering surgery has a role in uncontrolled primary angle-closure disease, whereas microinvasive glaucoma surgery combined with phacoemulsification offers promising results in selected cases of primary angle-closure glaucoma, facilitating a stepwise approach. This issue of Ophthalmology Rounds discusses these latest advances in our understanding of optimal imaging and management of patients with angle closure.
Angle closure describes an anatomical configuration where the peripheral iris mechanically blocks the trabecular meshwork (TM) through apposition and/or consequent to peripheral anterior synechiae (PAS).
Staging and Pathophysiology (Table 1)
Table 1. Staging of primary angle-closure disease
Primary angle closure suspect (PACS) is defined by the presence of ≥2 quadrants of iridotrabecular contact (ITC) but no signs of elevated intraocular pressure (IOP), PAS, or glaucoma.¹⁻³ Primary angle closure (PAC) is defined as the presence of ITC with elevated IOP and/or PAS in the absence of any glaucomatous optic neuropathy. Primary angle closure glaucoma (PACG) is defined as ITC that results in glaucomatous optic neuropathy.¹⁻³ While the majority of glaucomatous progression is from intermittent asymptomatic angle closure (i.e., chronic), acute angle-closure crisis (AACC) describes sudden and dramatic IOP elevation due to total TM occlusion.³⁻⁴ If detected and managed early, these episodes can allow definitive treatment of the angle closure before it leads to irreversible blindness.
PACG is a less common but more aggressive and blinding disease than primary open-angle glaucoma (POAG) .⁵⁻⁷ The prevalence of PACG is higher in people of Asian and Inuit origin and a high index of suspicion for the disease should be maintained in these populations.⁵⁺⁸ Risk factors for angle-closure disease include older age, female gender, shallow central and peripheral anterior chamber, positive family history for angle closure, shorter axial lengths and thicker crystalline lenses.⁹⁻¹² Hyperopia is not consistently associated with angle-closure disease, as cases of angle closure occur in people with a myopic refraction. In such cases, plateau iris is the usual mechanism.⁴
It is important to understand the pathophysiology of angle closure as this impacts the approach to management. The level of obstruction in aqueous circulation can vary. Obstruction most commonly occurs due to pupillary block.¹³ It can also be related to anomalies at the level of the ciliary body (plateau iris configuration) or due to an abnormal lens (lens-induced angle closure due to intumescence or zonulopathy). It is not uncommon for these mechanisms to coexist. Retrolental causes are uncommon but should be considered in selected cases such as malignant glaucoma, tumours, and uveal effusions that can be drug induced; e.g., topiramate.²⁺⁴⁺⁹⁺¹⁴
Imaging
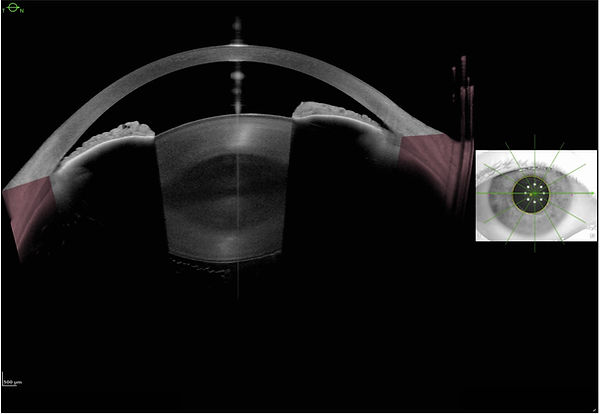
Anterior-segment (AS) imaging enables clinicians to investigate the mechanism of angle closure more accurately. AS optical coherence tomography (AS-OCT) is a highly sensitive, rapid, and contact-free method of detecting angle closure when compared to gonioscopy (Figure 1).¹⁵ AS-OCT can be performed in lower light conditions, thereby revealing unrecognized angle-closure disease.⁴ A systematic review and meta-analysis confirmed the sensitivity of AS-OCT, corresponding to a strong negative predictive value; however, a high false-positive rate indicates poor specificity.¹⁶ It is unclear whether false-positive results that refer to angles that appear to be closed on AS-OCT but not on gonioscopy represent errors and not simply eyes at higher risk of developing angle closure.
Figure 1. A case of iridotrabecular contact (ITC) in the temporal quadrant and significant narrowing of the angle nasally on anterior-segment optical coherence tomography (AS-OCT) in the presence of lens rise and pupillary block.
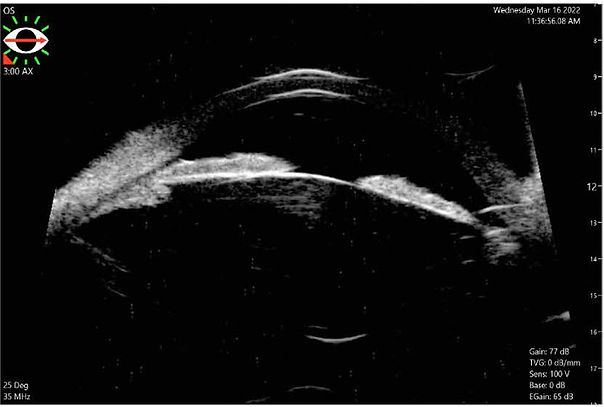
High-frequency ultrasound biomicroscopy (UBM) is useful to visualize the structures behind the iris, which is the main limitation of AS-OCT.¹⁴ UBM has been well established for more than 3 decades in the detection of a plateau iris configuration and is effective in the detection of pupillary block and malignant glaucoma (Figures 2A-C).¹⁷⁻¹⁹
Gonioscopy continues to be very important in diagnosing angle-closure disease. Dynamic gonioscopy enables detection of the presence of PAS towards establishment of the underlying mechanism.
Figures 2A-C. Pre- and post-laser peripheral iridotomy (LPI) AS-OCT images of a patient with ITC in the context of plateau iris configuration with concurrent pupillary block. It can be noted that the angle configuration and ITC did not improve post-LPI but the pupillary block resolved.
Figure 2A. The rectangular areas in yellow highlight the area of ITC and the arrow shows the pupillary block with the increased convexity of the iris at the pupillary margin.

Figure 2B. Appearances post-LPI with ongoing ITC contact highlighted in yellow but resolution of the pupillary block.

Figure 2C. The respective ultrasound biomicroscopy image better highlights the anterior position of the ciliary body and the difference in resolution between the 2 imaging modalities.
Management Considerations
The treatment of patients with angle-closure disease depends on the type of the angle closure, as described above. Recent landmark studies such as the ZAP trial and EAGLE study have changed the management of angle closure.⁷⁺²⁰
General management principles include treatment of fluctuations in the IOP, reduction of acute IOP increases, and relief of pupillary block.²¹ Laser peripheral iridotomy (LPI) and/or medical treatment have traditionally been used to treat angle closure since the mid-1970s.²² LPI as the treatment of choice is based on the hypothesis that relief of pupillary block and subsequent normalization of the pressure gradient between the posterior and the anterior chamber will result in posterior movement of the iris away from the TM.¹¹ Imaging with Scheimpflug photography in patients with occuladable angles has confirmed that the anterior chamber volume and angle increase post LPI. ²³
PACS
The Canadian Ophthalmological Society (COS) Glaucoma Clinical Practice Guidelines recommend the use of LPI for eyes with narrow angles at risk for an attack of acute angle closure; i.e., any degree of appositional closure, when >180° of TM cannot be visualized with proper gonioscopic maneuvres, or when the TM can be visualized for 360° but the approach is very narrow.⁸ The recent United Kingdom (UK) Royal College of Ophthalmologists (RCOphth) guidelines recommend LPI for people with PACS and additional risk factors such as a positive family history of angle closure, high hypermetropia (>6D), monocular vision, taking medications with anticholinergic activity, frequent pupillary dilatation, and difficulty accessing emergency ophthalmic care.⁴ This recommendation stemmed from the results of the ZAP trial, in which 889 patients with bilateral PACS received LPI in one eye and the contralateral eye served as an untreated control.²⁰ The primary outcome was incident PAC as a composite endpoint of IOP elevation, peripheral anterior synechiae, or acute angle closure during 72 months of follow-up. LPI significantly reduced the relative risk of developing PAC compared with no treatment (4.19 vs. 7.97 per 1000 eye-years; hazard ratio 0.53; 95% confidence interval [CI] 0.30-0.92; P=0.024). However, due to the low incidence of PAC in treated and untreated eyes in ZAP, prophylactic LPI for patients with PACS is not recommended. The European Glaucoma Society Guidelines (EGS) also recommend LPI in patients with PACS and high-risk features such as frequent pupil dilatation, positive family history of angle closure, and very high hyperopia.²
PAC and PACG
LPI
LPI has been the mainstay of treatment for cases of PAC and PACG for decades.²⁴ Nevertheless, an analysis of the use of LPI in PAC published by the American Academy of Ophthalmology (AAO) in 2018 showed that even though LPI results in an increase in angle width in all stages of primary angle closure, many PAC and AACC eyes and most PACG eyes require further treatment to control IOP.²⁵
Argon laser peripheral iridoplasty (ALPI)
A 2021 Cochrane review concluded that, despite the positive impact of ALPI on the anterior chamber morphology, it confers no additional benefit to the use of LPI alone in reducing IOP and preventing the progression of the disease.²⁶
Phacoemulsification
Evidence demonstrating the utility of phacoemulsification for cataract extraction in PACG was first published in the early 2000s.²⁷ A 2015 AAO report on the effects of phacoemulsification on longer-term IOP in different types of glaucoma, including 12 studies (N=495) of PACG, showed reductions in IOP and need for glaucoma medications of 30% and 58%, respectively, over a mean follow-up of 16 months.²⁷ A Bayesian analysis by Thomas et al showed that phacoemulsification in PACG has a 50% chance of reducing IOP by ≥5mm Hg.²⁸
Clear lens extraction (CLE)
The 2016 EAGLE trial was the first randomized, controlled trial comparing CLE with standard-of-care LPI in patients with PAC and IOP ≥30 mmHg or PACG.⁷ EAGLE was a large multicentre study that included 419 patients aged ≥50 years with a diagnosis of PAC and an IOP ≥30 mmHg or a diagnosis of PACG. Patients with symptomatic cataracts or advanced glaucoma (defined as MD worse than -15 db or cup-to-disc ratio ≥0.9) were excluded. Co-primary endpoints were IOP, patient-reported health status (European Quality of Life-5 Dimensions questionnaire), and incremental cost-effectiveness ratio per quality-adjusted life-year gained at 36 months post-treatment. Although EAGLE showed only a 1-mmHg difference in the mean IOP at 3 years, the authors commented that large differences were not to be expected as the study allowed clinicians to escalate treatment to achieve the target IOP (15-20 mmHg, depending on degree of optic nerve damage). Of greater clinical relevance is that only 21% of the participants in the CLE needing further treatment to control their IOP compared to 61% in the LPI group. Patient health status significantly favoured the CLE group and a high likeliness of cost-effectiveness was also determined for CLE vs. LPI.
It is worth noting though that cataract surgery in eyes with PAC or PACG can be more challenging compared to normal eyes due to the presence of a shallow anterior chamber, often a large lens and an atonic pupil.²³ In the EAGLE study the posterior capsule rupture rate was 1% similar to published cataract studies but the surgeries were performed by experienced surgeons with glaucoma subspecialty training. It is known that shorter axial length below 21 mm is associated with a higher complication rate.²⁹ As well, biometry measurements can be inaccurate and lead to refractive surprises.⁴
Combined phacoemulsification with trabeculectomy
The effect of combined phacotrabeculectomy was compared with phacoemulsification alone in parallel randomized, controlled studies in patients with medically controlled PACG and coexisting cataract and in those with medically uncontrolled PACG and cataract.³⁰⁻³¹ The former study found no significant difference in mean postoperative IOP at 24 months when 7 cases of hypotony were excluded from the phacotrabeculectomy group; however, that group required an average of 0.8 fewer topical drugs over 24 months compared to those who received phacoemulsification alone. As expected, the phacotrabeculectomy group experienced more complications compared to phacoemulsification alone.³⁰ The second study involving patients with medically uncontrolled PACG concluded that phacotrabeculectomy was significantly more effective compared to phacoemulsification alone in these patients at 15 and 18 months but not at 24 months, albeit with a higher number of complications.³¹ Patients who underwent phacotrabeculectomy required an average of 1.25 fewer topical drugs over 24 months. Thus, combined phacotrabeculectomy should be considered in patients with medically uncontrolled PACG and cataract.
Microinvasive glaucoma surgery (MIGS)
MIGS is defined as a group of minimally traumatic surgical approaches that are associated with good efficacy, high safety profile, and rapid recovery.³² The majority of MIGSs are performed ab-interno, in combination with cataract surgery, and spare the conjunctiva, allowing a stepwise approach that was impossible a decade ago. As explained above, some patients with PACG continue to experience elevated IOP after phacoemulsification despite the associated release of ITC with this procedure (except in cases of established PAS), thereby revealing an underlying trabecular dysfunction. Histological studies have shown that persistent ITC or PAS can cause progressive changes in the Schlemm canal and TM.³³
Over the last few years, several publications have presented favourable results for combined phacoemulsification and MIGS (phaco-MIGS) in patients with PACG. Chen et al showed that phacoemulsification with concurrent iStent® injection was associated with a higher likelihood of complete success (87.5%; 95% CI 58.6-96.7%) compared to phacoemulsification alone (43.8%; 95% CI 19.8-65.6%) at 12 months post-operatively (P=0.01).³⁴ Complete success was defined as IOP between 6-18 mmHg, no reoperations, and no IOP lowering medications required. Similar results have been reported in Canada and in Singapore in retrospective observational studies.³⁵⁻³⁶ The use of Kahook Dual Blade® or gonioscopy-assisted transluminal trabeculotomy (GATT) with or without goniosynechialysis but combined with phacoemulsification also yielded promising results in producing significant IOP reductions and/or reduction in postoperative IOP medications in patients with PACG.37,38 The main complications include hyphemas and/or IOP spikes in the early postoperative period; however, the majority are transient and can be treated conservatively.
AACC
Medical treatment
The main principles in managing an AACC are to lower the IOP medically, establish the diagnosis, and provide analgesia and antiemetics as required to relieve the associated symptoms of severe pain and/or nausea and vomiting.3,4 Due to the nature of AACCs, robust management data from randomized, controlled studies are limited.⁴ A large retrospective case series in Singapore found that medical therapy resulted in resolution of AACC within 12 hours in 76.2% of subjects and within 24 hours in 89.2%.³⁹
Medical treatment typically includes topical, oral, or intravenous carbonic anhydrase inhibitors (CAIs), topical a2-adrenergic agonists, topical parasympathomimetics, topical steroids, and oral or intravenous hyperosmotic agents such as mannitol.²⁻⁴ Topical medications may be ineffective initially due to the ciliary body and iris ischemia. Caution should be used with intensive administration of topical pilocarpine due to its systemic adverse events. Pilocarpine can worsen the acute attack in cases of a lenticular or retrolenticular cause of angle closure by shallowing the anterior chamber due to its effect on the ciliary muscle. In such cases, topical cycloplegia should be used instead. Marked anterior chamber asymmetry is an indicator of a phacomorphic element. It is also advised to verify whether topiramate is being used, as ciliary body effusions or bilateral angle closure secondary to topiramate can be exacerbated by pilocarpine.⁴
Acetazolamide is widely used to control acute IOP elevations;⁴ however, it has been associated with serious side effects such as Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), and aplastic anemia, particularly among patients of Korean and Japanese descent.⁴⁰⁻⁴¹ A large (N=128 942) longitudinal cohort study in Ontario assessed consecutive patients older than 65 years who were prescribed an oral or topical CAI (92.4% acetazolamide), primarily for glaucoma.⁴² The risks of experiencing the primary endpoint (SJS, TEN, or aplastic anemia) were 2.90 and 2.08 per 1000 patients with oral or topical administration, respectively. The risks of cardiac dysrhythmia (100.77 per 1000 patients) and acute kidney injury were substantial (13.23 per 1000 patients). This study did not assess whether a previous sulfonamide allergy increases the risk of a reaction to CAIs. Retrospective analyses have found that acetazolamide appears well tolerated even in patients predisposed to SJS/TEN or with a self-reported sulfonamide allergy.⁴³⁻⁴⁴
LPI
LPI is recommended for an AACC whenever technically possible; i.e., the cornea is clear enough to facilitate the LPI.²⁻⁴⁺⁸ Studies have shown that nearly half of patients undergoing LPI developed elevated IOP within 6 months of the AACC that necessitated further medical and/or surgical treatment.⁴⁵⁻⁴⁶ LPI is associated with IOP spikes (6%-10%), dysphotopsia (2%-11%), anterior chamber bleeding (30-41%), cataract progression (23%-39% over 1-6 years), and endothelial cell loss.²⁵⁺⁴⁷⁺⁴⁸ Post-LPI inflammation was reported in only 0.5% of eyes in the LPI arm of the EAGLE study;⁷ however, it can be potentiated in an already inflamed eye with AACC.
Contralateral fellow eyes
The risk of AACC is elevated in contralateral fellow eyes, and up to 50% may develop AACC within years.³⁺⁴⁹⁻⁵⁰ It is recommended that prophylactic LPI be performed in fellow eyes.²⁻⁴ A retrospective case series of 80 fellow eyes that underwent prophylactic LPI found that none developed AACC in the 4 years of follow-up.⁴⁵ Conversely, an observational retrospective case series of 114 Caucasian eyes in Italy found that almost half of fellow eyes developed chronic angle closure even in the presence of a prophylactic iridotomy.⁵¹ For this reason, it is important to consider lens-based surgery in fellow eyes to an AACC.
Anterior chamber paracentesis (ACP)
ACP can be an effective method of resolving an angle closure attack as it can rapidly lower the IOP, clear the cornea, relieve any associated pain and nausea, and facilitate an LPI where appropriate.²⁺⁵² COS, AAO, and EGS guidelines support the use of ACP for cases of refractory AAAC or when LPI is impossible.²⁻³⁺⁸ This can be performed using a 30-gauge needle under aseptic technique and appropriate topical anesthesia in the slit lamp. It is advised to make a long track parallel to the iris to prevent leakage and shallowing of the chamber. Complications may include damage to the surrounding iris, lens, or corneal endothelium, malignant glaucoma, suprachoroidal hemorrhage, decompression retinopathy, hyphema, and endophthalmitis.⁵² A small case series by Lam et al looked at 8 consecutive patients diagnosed with AAAC and an IOP at presentation >50 mmHg who received topical treatment, immediate ACP, and systemic acetazolamide and mannitol.⁵³ They observed a rapid reduction in the IOP that was sustained to 2 hours (average 20.1 mm Hg) and rapid symptomatic relief in all patients with no complications. Arnavielle et al prospectively evaluated ACP use in 14 patients with AACC and 6 with secondary glaucoma.⁵⁴ Mean IOP decreased from 53.4±4.2 mmHg at baseline to 18.2±11.1 mmHg at 2 hours with no reported complications. At 7 days post-ACP, mean IOP was 16.4±10.7 mmHg.
ALPI and cyclodiode laser
Several studies have investigated the usefulness of ALPI for the treatment of AACC.⁵⁵⁻⁵⁷ Application of photocoagulation burns to the surface of the peripheral iris causes focal contraction of the iris tissue and mechanical pulling away from the TM.¹⁴ The RCOphth guidelines on angle closure recommend the use of ALPI if there is insufficient response within 2 hours of LPI or LPI is technically impossible to perform due to corneal edema.⁴
The use of cyclodiode laser in the management of AACC has been described in small observational studies in the UK and it is recommended by the RCOphth guidelines for refractory cases. Cyclodiode laser appears to be effective in resolving the attack within 1 day with a good safety profile and can prepare the eye for subsequent lens extraction.⁵⁸⁻⁶⁰ Reported complications in these studies included low-grade inflammation, self-limiting ciliochoroidal detachment, and hyphema.
Phacoemulsification
An analysis of 4 studies (N=119) that evaluated phacoemulsification for the management of AACC determined that the mean IOP decreased from 50.1 mmHg to 14.7 mmHg after a mean follow-up of 2 years with 0.1 medications.²⁷ Two of these studies looked at the efficacy of early phacoemulsification vs. laser peripheral iridotomy (LPI). In the study by Lam et al,⁶² Chinese patients were randomized to early phacoemulsification or LPI within days after medical resolution of the AACC.⁴⁶ At 18 months, 3.2% of patients in the phacoemulsification group experienced an increase in IOP above 21 mmHg compared to 46.7% of patients in the LPI group (P<0.0001). Mean IOP was significantly lower in the phacoemulsification group than with LPI (12.6±1.9 mmHg vs. 15.0±3.4 mmHg; P=0.009), and significantly fewer medications were required to maintain IOP ≤21 mmHg in the phacoemulsification group than the LPI group (0.03±0.18 vs. 0.90±1.14; P<0.0001). The second study, from the UK, randomized 37 patients who had responded to medical treatment for AACC to receive LPI or phacoemulsification with implantation of an intraocular lens (phaco/IOL) within 1 week of presentation.⁶¹ The primary outcome measure was failure to control IOP, defined as IOP 22-24 mmHg on 2 occasions (readings taken within 1 month of each other) or ≥25 mmHg on 1 occasion, after week 3. The 2-year cumulative survival rates were 89.5% in the phaco/IOL group and 61.1% in the LPI group. In terms of complications, in the LPI group there was 1 transient hemorrhage, 1 corneal burn, and in 3 cases the LPI had to be repeated because of non-patency whereas in the phaco/IOL group the only complication was an IOP of 30 mmHg on day 1 for 1 patient. The authors concluded that early phacoemulsification and IOL implantation should be considered in cases of AACC that are controlled medically with the caveat that it should be performed by experienced surgeons.
Conclusions
Our understanding of angle-closure disease has improved significantly over the last few decades. Studies have shown that phacoemulsification as an alternative to LPI is central to the management of PACG. Combined phacoemulsification and filtering surgery is indicated in medically uncontrolled PACG. Phacoemulsification combined with goniosynechialysis or a TM bypass offers promising results in reducing both IOP and/or the number of IOP medications, and this combination may facilitate a stepwise management approach in some cases of PACG. In patients with PAC, options include LPI vs. phacoemulsification with the latter being potentially more beneficial in specific cases of PAC. LPI is recommended in select patients with high-risk PACS, while LPI or observation are options for low-risk cases. In AACC, medical treatment is the mainstay of treatment with some cases requiring an ACP to resolve the attack. This is followed by lens-based surgery with or without goniosynechialysis, TM bypass, or filtering surgery. LPI might not always be the most appropriate initial treatment option, especially in severely inflamed eyes, shallow anterior chambers, or with a suboptimal view of the anterior chamber. Fellow eyes in cases of AAC will benefit from LPI or lens-based surgery.
References
-
Foster PJ, Buhrmann R, Quigley HA, Johnson GJ. The definition and classification of glaucoma in prevalence surveys. Br J Ophthalmol. 2002;86(2):238-242.
-
European Glaucoma Society Terminology and Guidelines for Glaucoma, 5th Edition. Br J Ophthalmol. 2021;105(Suppl 1):1-169.
-
American Academy of Ophthalmology. Preferred Practice Pattern® Guidelines. Primary Angle Closure 2020. Available at: https://www.aao.org/education/preferred-practice-pattern/primary-angle-closure-disease-ppp. Accessed July 9, 2023.
-
Royal College of Ophthalmologists. Clinical Guidelines. The Management of Angle Closure Glaucoma. Available at: https://www.rcophth.ac.uk/wp-content/uploads/2022/06/Management-of-Open-Angle-Closure-Glaucoma-1.pdf. Accessed July 9, 2023.
-
Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90(3):262-267.
-
George R, Panda S, Vijaya L. Blindness in glaucoma: primary open-angle glaucoma versus primary angle-closure glaucoma-a meta-analysis. Eye (Lond). 2022;36(11):2099-2105.
-
Azuara-Blanco A, Burr J, Ramsay C, et al; The EAGLE Study Group. Effectiveness of early lens extraction for the treatment of primary angle-closure glaucoma (EAGLE): a randomised controlled trial. Lancet. 2016;388(10052):1389-1397.
-
Canadian Ophthalmological Society Glaucoma Clinical Practice Guideline Expert Committee; Canadian Ophthalmological Society. Canadian Ophthalmological Society evidence-based clinical practice guidelines for the management of glaucoma in the adult eye. Can J Ophthalmol. 2009;44(Suppl 1):S7-S93. Erratum in: Can J Ophthalmol. 2009;44(4):477.
-
Quigley HA, Friedman DS, Congdon NG. Possible mechanisms of primary angle-closure and malignant glaucoma. J Glaucoma. 2003;12(2):167-180.
-
Amerasinghe N, Aung T. Angle-closure: risk factors, diagnosis and treatment. Prog Brain Res. 2008;173:31-45.
-
Tarongoy P, Ho CL, Walton DS. Angle-closure glaucoma: the role of the lens in the pathogenesis, prevention, and treatment. Surv Ophthalmol. 2009;54(2):211-225.
-
Amerasinghe N, Zhang J, Thalamuthu A, He M, Vithana EN, Viswanathan A, Wong TY, Foster PJ, Aung T. The heritability and sibling risk of angle closure in Asians. Ophthalmology. 2011;118(3):480-485.
-
Weinreb RN, Aung T, Medeiros FA. The pathophysiology and treatment of glaucoma: a review. JAMA. 2014 May 14;311(18):1901-11
-
Wright C, Tawfik MA, Waisbourd M, Katz LJ. Primary angle-closure glaucoma: an update. Acta Ophthalmol. 2016;94(3):217-225.
-
Nolan WP, See JL, Chew PT, et al. Detection of primary angle closure using anterior segment optical coherence tomography in Asian eyes. Ophthalmology. 2007;114(1):33-39.
-
Desmond T, Tran V, Maharaj M, Carnt N, White A. Diagnostic accuracy of AS-OCT vs gonioscopy for detecting angle closure: a systematic review and meta-analysis. Graefes Arch Clin Exp Ophthalmol. 2022;260(1):1-23. Erratum in: Graefes Arch Clin Exp Ophthalmol. 2022;260(1):385.
-
Pavlin CJ, Ritch R, Foster FS. Ultrasound biomicroscopy in plateau iris syndrome. Am J Ophthalmol. 1992;113(4):390-395.
-
Aslanides IM, Libre PE, Silverman RH, et al. High frequency ultrasound imaging in pupillary block glaucoma. Br J Ophthalmol. 1995;79(11):972-976.
-
Trope GE, Pavlin CJ, Bau A, Baumal CR, Foster FS. Malignant glaucoma. Clinical and ultrasound biomicroscopic features. Ophthalmology. 1994;101(6):1030-1035.
-
He M, Jiang Y, Huang S, et al. Laser peripheral iridotomy for the prevention of angle closure: a single-centre, randomised controlled trial. Lancet. 2019;393(10181):1609-1618.
-
Saw SM, Gazzard G, Friedman DS. Interventions for angle-closure glaucoma: an evidence-based update. Ophthalmology. 2003;110(10):1869-1878.
-
Robin AL, Pollack IP. Argon laser peripheral iridotomies in the treatment of primary angle closure glaucoma. Arch Ophthalmol. 1982;100(6):919-923.
-
Talajic JC, Lesk MR, Nantel-Battista M, Harasymowycz PJ. Anterior segment changes after pilocarpine and laser iridotomy for primary angle-closure suspects with Scheimpflug photography. J Glaucoma. 2013;22(9):776-779.
-
Prum BE Jr, Rosenberg LF, Gedde SJ, et al. Primary open-angle glaucoma Preferred Practice Pattern(®) Guidelines. Ophthalmology. 2016;123(1):P41-P111. Erratum in: Ophthalmology. 2018;125(6):949.
-
Radhakrishnan S, Chen PP, Junk AK, Nouri-Mahdavi K, Chen TC. Laser Peripheral Iridotomy in Primary Angle Closure: A Report by the American Academy of Ophthalmology. Ophthalmology. 2018;125(7):1110-1120.
-
Bayliss JM, Ng WS, Waugh N, Azuara-Blanco A. Laser peripheral iridoplasty for chronic angle closure. Cochrane Database Syst Rev. 2021;3(3):CD006746.
-
Chen PP, Lin SC, Junk AK, Radhakrishnan S, Singh K, Chen TC. The effect of phacoemulsification on intraocular pressure in glaucoma patients: a report by the American Academy of Ophthalmology. Ophthalmology. 2015;122(7):1294-1307.
-
Thomas R, Walland M, Thomas A, Mengersen K. Lowering of intraocular pressure after phacoemulsification in primary open-angle and angle-closure glaucoma: a Bayesian analysis. Asia Pac J Ophthalmol (Phila). 2016;5(1):79-84.
-
Day AC, MacLaren RE, Bunce C, Stevens JD, Foster PJ. Outcomes of phacoemulsification and intraocular lens implantation in microphthalmos and nanophthalmos. J Cataract Refract Surg. 2013;39(1):87-96.
-
Tham CC, Kwong YY, Leung DY, et al. Phacoemulsification versus combined phacotrabeculectomy in medically controlled chronic angle closure glaucoma with cataract. Ophthalmology. 2008;115(12):2167-2173.e2.
-
Tham CC, Kwong YY, Leung DY, et al. Phacoemulsification versus combined phacotrabeculectomy in medically uncontrolled chronic angle closure glaucoma with cataracts. Ophthalmology. 2009;116(4):725-731.
-
Saheb H, Ahmed II. Micro-invasive glaucoma surgery: current perspectives and future directions. Curr Opin Ophthalmol. 2012;23(2):96-104.
-
Hamanaka T, Kasahara K, Takemura T. Histopathology of the trabecular meshwork and Schlemm’s canal in primary angle-closure glaucoma. Invest Ophthalmol Vis Sci. 2011;52(12):8849-8861.
-
Chen DZ, Sng CCA, Sangtam T, et al. Phacoemulsification vs phacoemulsification with micro-bypass stent implantation in primary angle closure and primary angle closure glaucoma: A randomized single‑masked clinical study. Clin Exp Ophthalmol. 2020;48(4):450-461.
-
Salimi A, Abu-Nada M, Harasymowycz P. Matched cohort study of cataract surgery with and without trabecular microbypass stent implantation in primary angle-closure glaucoma. Am J Ophthalmol. 2021;224:310-320.
-
Hernstadt DJ, Cheng J, Htoon HM, Sangtam T, Thomas A, Sng CCA. Case series of combined iStent implantation and phacoemulsification in eyes with primary angle closure disease: one-year outcomes. Adv Ther. 2019;36(4):976-986.
-
Dorairaj S, Tam MD, Balasubramani GK. Twelve-month outcomes of excisional goniotomy using the Kahook Dual Blade® in eyes with angle-closure glaucoma. Clin Ophthalmol. 2019;13:1779-1785.
-
Sharkawi E, Artes PH, Lindegger DJ, et al. Gonioscopy-assisted transluminal trabeculotomy in primary angle-closure glaucoma. Graefes Arch Clin Exp Ophthalmol. 2021;259(10):3019-3026.
-
Ramli N, Chai SM, Tan GS, Husain R, Hoh ST, Ho CL, Aung T. Efficacy of medical therapy in the initial management of acute primary angle closure in Asians. Eye (Lond). 2010;24(10):1599-1602.
-
Schuman JS. Antiglaucoma medications: a review of safety and tolerability issues related to their use. Clin Ther. 2000;22(2):167-208.
-
Her Y, Kil MS, Park JH, Kim CW, Kim SS. Case report: Stevens-Johnson syndrome induced by acetazolamide. J Dermatol. 2011;38(3):272-275.
-
Popovic MM, Schlenker MB, Thiruchelvam D, Redelmeier DA. Serious adverse events of oral and topical carbonic anhydrase inhibitors. JAMA Ophthalmol. 2022;140(3):235-242.
-
Kumar R, Dohlman CH, Chodosh J. Oral acetazolamide after Boston keratoprosthesis in Stevens Johnson syndrome. BMC Res Notes. 2012;5:205.
-
Lee AG, Anderson R, Kardon RH, Wall M. Presumed “sulfa allergy” in patients with intracranial hypertension treated with acetazolamide or furosemide: cross-reactivity, myth or reality? Am J Ophthalmol. 2004;138(1):114-118.
-
Aung T, Ang LP, Chan SP, Chew PT. Acute primary angle-closure: long-term intraocular pressure outcome in Asian eyes. Am J Ophthalmol. 2001;131(1):7-12.
-
Lam DS, Leung DY, Tham CC, et al. Randomized trial of early phacoemulsification versus peripheral iridotomy to prevent intraocular pressure rise after acute primary angle closure. Ophthalmology. 2008;115(7):1134-1140.
-
Waisbourd M, Shafa A, Delvadia R, Sembhi H, Molineaux J, Henderer J, Pizzi LT, Myers JS, Hark LA, Katz LJ. Bilateral Same-day Laser Peripheral Iridotomy in the Philadelphia Glaucoma Detection and Treatment Project. J Glaucoma. 2016;25(10):e821-e825.
-
Park HY, Lee NY, Park CK, Kim MS. Long-term changes in endothelial cell counts after early phacoemulsification versus laser peripheral iridotomy using sequential argon:YAG laser technique in acute primary angle closure. Graefes Arch Clin Exp Ophthalmol. 2012;250(11):1673-1680.
-
Lowe RF. Acute angle-closure glaucoma: the second eye: an analysis of 200 cases. Br J Ophthalmol. 1962;46(11):641-650.
-
Snow JT. Value of prophylactic peripheral iridectomy on the second eye in angle-closure glaucoma. Trans Ophthalmol Soc UK. 1977;97(1):189-191.
-
Fea AM, Dallorto L, Lavia C, Pignata G, Rolle T, Aung T. Long-term outcomes after acute primary angle closure of Caucasian chronic angle closure glaucoma patients. Clin Exp Ophthalmol. 2018;46(3):232-239.
-
Boey PY, Singhal S, Perera SA, Aung T. Conventional and emerging treatments in the management of acute primary angle closure. Clin Ophthalmol. 2012;6:417-24.
-
Lam DS, Chua JK, Tham CC, Lai JS. Efficacy and safety of immediate anterior chamber paracentesis in the treatment of acute primary angle-closure glaucoma: a pilot study. Ophthalmology. 2002;109(1):64-70.
-
Arnavielle S, Creuzot-Garcher C, Bron AM. Anterior chamber paracentesis in patients with acute elevation of intraocular pressure. Graefes Arch Clin Exp Ophthalmol. 2007;245(3):345-350.
-
Fu J, Qing GP, Wang NL, Wang HZ. Efficacy of laser peripheral iridoplasty and iridotomy on medically refractory patients with acute primary angle closure: a three year outcome. Chin Med J (Engl). 2013;126(1):41-45.
-
Lai JS, Tham CC, Chua JK, Poon AS, Lam DS. Laser peripheral iridoplasty as initial treatment of acute attack of primary angle-closure: a long-term follow-up study. J Glaucoma. 2002;11(6):484-487.
-
Lam DS, Lai JS, Tham CC, Chua JK, Poon AS. Argon laser peripheral iridoplasty versus conventional systemic medical therapy in treatment of acute primary angle-closure glaucoma: a prospective, randomized, controlled trial. Ophthalmology. 2002;109(9):1591-1596.
-
Manna A, Foster P, Papadopoulos M, Nolan W. Cyclodiode laser in the treatment of acute angle closure. Eye (Lond). 2012;26(5):742-745.
-
Chiam PJ, Sung VCT. The outcome of transscleral cyclophotocoagulation for the management of acute angle closure. Eur J Ophthalmol. 2018;28(2):188-192.
-
Liu W, Qin L, Xu C, et al. Transscleral cyclophotocoagulation followed by cataract surgery: a novel protocol to treat refractory acute primary angle closure. BMC Ophthalmol. 2020;20(1):209.
-
Husain R, Gazzard G, Aung T, et al. Initial management of acute primary angle closure: a randomized trial comparing phacoemulsification with laser peripheral iridotomy. Ophthalmology. 2012;119(11):2274-2281.
Financial Disclosures:
Drs. Siempis and Schlenker stated that they have no disclosures to report in association with the contents of this issue.
Dr. Siempis is a Clinical Fellow in Glaucoma and Advanced Anterior Segment Surgery, Prism Eye Institute, University Health Network, Trillium Health Partners, University of Toronto, Toronto, Ontario. Dr. Schlenker is an Associate Professor, Department of Ophthalmology and Vision Sciences, University of Toronto, and a Glaucoma, Cataract, and Advanced Anterior Segment Surgeon, University Health Network, Kensington Eye Institute, and Trillium Health Partners, Toronto, Ontario.
Ophthalmology Rounds is made possible through educational funding
from the following industry co-sponsors:
Partner: Biogen
Supporters: AbbVie, Apellis, Bayer, Roche, Teva