
Surgical Techniques and Management Strategies for Patients
With Acute Anterior Segment Trauma
By Marcela Huertas-Bello, MD, Mor Bareket, MD, and Clara C. Chan, MD, FRCSC
Anterior segment trauma encompasses a broad spectrum of injuries that can significantly impact vision and ocular health if not promptly and appropriately managed. A systematic and meticulous approach is essential to accurately diagnose and address the underlying injury while minimizing operative considerations, systems for classification of ocular trauma, and surgical and supportive nonsurgical repair of various types of anterior segment trauma.
Ocular injuries represent a critical threat to vision and are a leading cause of preventable monocular blindness and visual impairment worldwide. During 2012–2017 in Ontario, 256 776 patients presented to the emergency department with trauma-related conditions.¹ Notably, 51.0% of these cases were associated with cornea and anterior segment trauma. Furthermore, almost one-third of patients with severe eye trauma do not recover vision better than 20/200.²
General Principles for Approaching Anterior Segment Trauma
A thorough history to determine the type of object that caused the injury can help in differentiating a clean vs a dirty wound, ascertaining the mechanism of injury (blunt or penetrating), and if it is a work injury. Establishing if the patient was using protective eyewear and calculating the time since the trauma can assist with assessing the risk and potential microbial spectrum for secondary infection. A detailed history should include the patient’s ocular history, including prior conditions or surgeries (e.g., laser-assisted in situ keratomileusis [LASIK] or cataract surgery). Look for associated systemic injuries, such as facial fractures or head trauma, stabilize vital signs if the patient has concurrent life-threatening injuries.
An ophthalmological examination measuring visual acuity (VA) is essential to estimate visual prognosis, and an external inspection should be conducted to look for obvious signs of injury: lacerations, foreign bodies, or extruded intraocular contents. It is reasonable to avoid intraocular pressure (IOP) measurement and take caution to avoid the use of excessive force during examination. Table 1 summarizes key pearls to be attentive to on slit lamp examination that can suggest a ruptured globe.
Table 1. Key suspicious findings during the slit lamp examination
Computed tomography scan typically performed in the emergency department on patient arrival can help confirm globe rupture, foreign bodies, and orbital fractures. Thin slices (1-2 mm) provide better detail. Magnetic resonance imaging should be avoided as it is contraindicated if metallic foreign bodies are suspected.
Endophthalmitis rates after ocular trauma are reported to be up to 16.5%.³ Intracameral antibiotic prophylaxis has been shown to be effective to reduce the risk of endophthalmitis.⁴⁻⁶ For patients with contaminated, high-risk wounds who have an unknown tetanus vaccination history or have received fewer than 3 vaccinations in the series, it is recommended to administer 500 IU of tetanus immunoglobulin (TIG) and the tetanus toxoid vaccine.7 If TIG is unavailable, an alternative is to use 200–400 mg/kg of intravenous immunoglobulin.⁷
Surgical primary repair should be performed within 24 hours to minimize the risk of infection and enhance outcomes. Covering the traumatized eye with a rigid eye shield rather than a patch is useful to protect against any possible additional injury before the surgical procedure.⁸ Furthermore, the patient should be advised against Valsalva maneuvers, including coughing or sneezing.
General anesthesia is usually preferred for open-globe injury repair to provide full paralysis. It is also the recommended approach for children or uncooperative patients, for exploratory surgeries, or in situations where the severity of the injury cannot be assessed beforehand.⁹⁻¹⁰ Important considerations for general anesthesia include avoiding depolarizing blocking agents like succinylcholine as it may increase IOP by as much as 10 mm Hg, leading to extrusion of ocular contents.¹¹
Perioperative topical eye drop regimens are tailored to optimize outcomes, minimize complications, and promote healing after trauma repair. Fluoroquinolones (e.g., moxifloxacin) are commonly used to reduce bacterial flora and lower the risk of postoperative infections. Topical steroids are key in inflammation control. Cycloplegic agents relax the iris and ciliary body to reduce intraoperative inflammation and pain. The use of antiglaucoma medications postoperatively may be required as well since traumatic IOP elevation and traumatic glaucoma are complications that can result from the initial eye injury and surgical repair.¹²
Long-term management requires consistent follow-up to carefully monitor glaucoma, retinal detachment, and cataract formation. Discussion about the theoretical risk of sympathetic ophthalmia to the unaffected eye should also be carried out and documented.
Ocular Trauma Classification
Ocular trauma classification systems standardize the evaluation, communication, and management of traumatic eye injuries. They are crucial for estimating visual prognosis and effectively explaining it to the patient, which is also significant from a medicolegal aspect. The Birmingham Eye Trauma Terminology System (BETTS) is the most widely used system in the published literature (Figure 1).¹³
Figure 1. Birmingham Eye Trauma Terminology System (BETTS).¹³

Management of Anterior Segment Trauma
Conjunctival lacerations
Small lacerations (<10 mm) not involving the limbus or fornix can often heal without surgical intervention. Large lacerations (>10 mm) or fornix involvement require placing sutures to ensure proper alignment, restore the anatomy, and prevent scarring; absorbable sutures (e.g., 8-0 or 9-0 polyglactin 910) are recommended. Lacerations with limbal involvement require meticulous repair to prevent limbal stem cell deficiency.
Corneal burn injuries
Ocular surface burns can be caused by chemicals (alkali or acid), ultraviolet light, or direct heat. The impact of the inciting agent’s initial damage and physical properties determine the prognosis for visual recovery and healing of the ocular surface.¹⁴ Should significant damage occur to the limbal stem cells, there may be delayed re-epithelialization, permanent failure of stem cell function, and conjunctivalization of the cornea.¹⁵
Treatment of corneal burn injuries includes irrigation with a phosphate-free saline solution until the pH is neutralized, preservative-free topical antibiotic, corticosteroid, and cycloplegics. Systemic medications may include vitamin C and tetracyclines, which may help reduce the risk of corneal stromal ulceration by inhibiting matrix metalloproteinases.¹⁵
Corneal burn injuries typically result in a large corneal and conjunctival (inferior more often than superior due to Bell’s reflex) epithelial defect.¹⁶ A bandage contact lens with topical antibiotic prophylaxis can be used with or without a fresh frozen or dehydrated amniotic membrane graft to reduce inflammation and promote re-epithelialization. It can take weeks to months for full epithelial recovery. Should there be a neurotrophic component due to corneal nerve damage, a tarsorrhaphy may be helpful.
Prognosis can be determined by the Roper-Hall classification system (Table 2).¹⁷
Table 2. Grading ocular burns: classification of severity of ocular surface burns by Roper-Hall¹⁷
Corneal abrasion
In the context of management, ensure that all foreign bodies have been removed. Thorough examination of the upper eyelid is critical to identifying foreign objects. A Cochrane review found that treating simple corneal abrasions with a patch may not improve healing or reduce pain; also, patches can decrease oxygen delivery and promote infection.¹⁸ Bandage contact lenses effectively alleviate pain from traumatic abrasions while preserving vision during treatment, promoting epithelialization by forming a scaffold, and improving the spread of tear fluid over the ocular surface.¹⁹
The use of topical antibiotic prophylaxis to prevent ocular infection or accelerate epithelial healing following a corneal abrasion remains unclear. Moreover, the current evidence is insufficient to demonstrate the superiority of any one antibiotic regimen.²⁰ Despite controversies related to topical anesthetic use in patients with corneal injuries, they are prescribed frequently by emergency medicine physicians. The pain pathway is crucial for corneal healing, as pain signals from an injured cornea activate the trigeminal nerve, enhancing lubrication through increased blink reflex and tear production.²¹ This process delivers growth factors like insulin-like growth factor 1, substance P, and nerve growth factor that support epithelial nourishment and healing. Prolonged administration of topical analgesic agents impairs growth factor synthesis, leading to epithelial loss, abnormal neuronal growth, neuropathic pain, and fibrosis that manifests clinically as corneal scarring, persistent epithelial defects, stromal infiltrates, ring-shaped keratitis, and hypopyon, with high risk for superimposed infections such as bacterial and/or fungal keratitis.²²⁻²³
Recurrent corneal erosion syndrome is a complication following traumatic corneal abrasions, especially erosions caused by sharp objects such as fingernails, tree branches, mascara brushes, and paper cut injuries. It occurs due to poor epithelial adhesion to the underlying basement membrane, leading to repeated episodes of corneal epithelial breakdown. Conservative management options for acute flare-ups consist of lubricating eye drops, nightly ointment, and oral matrix metalloproteinase inhibitors.²⁴
Corneal foreign bodies
The goal is to safely remove the foreign bodies while minimizing damage to the cornea and prevent complications such as infection or scarring. Eversion of the upper eyelid is crucial for identifying any additional foreign materials. In the case of metallic foreign bodies, ensure that all rust rings are removed to prevent delayed healing and scarring (Figure 2). Special precautions are necessary for foreign body injuries involving organic matter (wood or plant material) or animal exposure (including scratches or contact with fecal material) due to the increased risk of infections. If an infection is suspected, obtain corneal scrapings for bacteria, fungi, and acanthamoeba cultures. Broad-spectrum topical antibiotics such as moxifloxacin are recommended as first-line options. In cases where a Pseudomonas infection is suspected, fortified tobramycin should be prescribed.⁷ Antifungal treatments such as natamycin 5%, voriconazole 1%, or amphotericin B drops should be administered if a fungal infection is suspected.
Figure 2. Corneal metallic foreign bodies and rust ring.

LASIK flap traumatic complications
LASIK eye surgery has been approved in Canada since the early 1990s with millions of patients treated since.²⁵ The LASIK flap is created using a microkeratome blade or the femtosecond laser. The LASIK flap typically has a superior hinge and adheres due to surface tension, epithelial adhesion, and endothelial pump function. There is limited stromal healing, and with sufficient blunt or sharp trauma, the flap is vulnerable to folds and striae, dislocations, or dehiscence. Macrostriae represents poor edge apposition that forms after flap trauma and requires repair by flap lift, hydration with balanced salt solution, and repositioning with potential suture fixation, or else it may lead to epithelial ingrowth. If the stromal bed is exposed due to a folded or dislocated LASIK flap (Figure 3), the flap should also be repositioned as soon as possible with a moistened cellulose sponge. If the flap is dehisced with tissue loss, a bandage contact lens can be placed to allow the epithelium to grow back to cover the exposed stroma. Unfortunately, the resulting irregular astigmatism may require a scleral contact lens. Topical antibiotic and steroid drops are used after flap repositioning to prevent infection and reduce the risk of inflammatory reactions in the flap interface.
Figure 3. Dislocated LASIK flap. Note the exposed stromal bed to the bottom right of the slit lamp light.
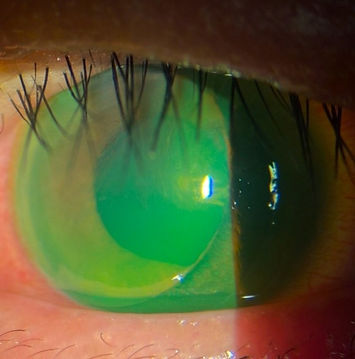
Corneal lacerations
Repairs should be performed within 24 hours to minimize the risk of infection, alleviate patient discomfort, and prevent further damage to the eye. The repair goals include achieving a watertight closure, restoring normal anatomy, preventing infection, promoting re-epithelialization and stromal healing, preventing high astigmatism and scarring, and ensuring the best possible outcome in preparation for a subsequent procedure.²⁶
Careful examination is essential to confirm the presence of a perforating lesion. The Seidel test, utilizing fluorescein, is particularly effective in detecting microscopic leaks.²⁷ Additionally, applying gentle digital pressure may help identify a self-sealing wound. Anterior segment optical coherence tomography can help document the injury and evaluate the depth. Figure 4 presents a management flowchart for corneal lacerations, proposed by Hersh et al.²⁸
Figure 4. Management flowchart for addressing corneal lacerations²⁸
Partial-thickness lacerations
Patching and bandage contact lenses provide structural support by acting as mechanical splints, which help prevent leakage in small lacerations or minor perforations.²⁹ Pressure patching may be a viable option for patients whose wound edges are well aligned and do not have any gaps; however, excess residual pressure from the tight patch can counteract its effectiveness.³⁰ Additionally, a challenge with using a patch is that it prevents assessment of the anterior chamber; therefore, if there is no improvement after 24 hours of patch use, it is advisable to consider an alternative therapeutic option.³¹ If the wound is unstable, a bandage soft contact lens may be used to support the wound and promote re-epithelialization.²⁸
Cyanoacrylate tissue adhesive is highly beneficial for lacerations that need more support than a bandage contact lens can offer. For the application, it is advisable to debride the necrotic tissue, dry the surface well, and avoid applying large quantities of glue since it can dislodge easily and will be uncomfortable for the patient.³²
Full-thickness lacerations
Surgery should be done as soon as possible to reduce the risk of expulsive hemorrhages and to reduce desiccation of prolapsed intraocular tissues (Figure 5).³² A dehisced corneal transplant may be treated like a full-thickness laceration for which primary closure should be attempted as soon as possible.
Figure 5. Full thickness corneal laceration into the visual axis. Note the corneal striae due to a flat anterior chamber, vitreous loss, and total hyphema. The lens was also dislocated with iris loss more evident after anterior chamber washout, lensectomy and anterior vitrectomy with 10-0 nylon interrupted sutures to close the corneal laceration.
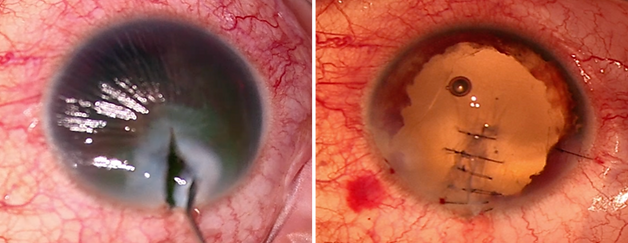
Surgical Principles for Corneal Sutures
Cleaning the edges of the wound is essential; preserve as much tissue as possible and take the time to “organize” the wounds while recognizing anatomic landmarks. Properly aligning these landmarks significantly simplifies the anatomical repair of the wound. If the landmarks are difficult to find or the wound edges do not align correctly, look for partially attached corneal fragments or lacerated corneal edges that may be tucked beneath the surrounding cornea.³²
Nylon 10-0 spatulated monofilament is a good option for corneal sutures because it produces minimal tissue inflammation, which helps reduce vascularization and scarring. Proper management of corneal sutures and ensuring that the knots are always buried are essential. Additionally, document the findings in detail and how the repair was performed for planning future surgeries.
Corneal sutures should be approximately 90%–95% depth through the stroma. Sutures with short trajectories are not recommended, as they can flatten the central cornea. It is advisable to use short sutures in the centre and longer sutures in the periphery. Additionally, the sutures take an equal amount of tissue from both edges. Use of 360º conjunctival peritomy for globe exploration is advisable, especially in cases of laceration at the limbus and behind extraocular muscles, which present the highest risk for globe rupture.
Linear laceration
The first suture should be placed in the centre of the laceration, and then additional sutures should bisect the halves until they are sealed.²⁶
Oblique laceration
Use care to place sutures that are an equal distance from the posterior wound edge. From the anterior corneal view, however, the sutures will look displaced. Slip knots can be helpful to control the tension over the shallow side of the wound when tying the suture.²⁶
Gaping laceration
Place the initial suture at the end of the wound that is least gaping and then advance along the wound, placing additional sutures to “zip up” the gape while using viscoelastic to keep internal tissues away from the closure.²⁶
Stellate laceration
Stellate lacerations of the cornea pose a complex surgical challenge because they feature multiple jagged edges that complicate the closure process. These irregular edges make it difficult to employ a straightforward strategy to bring them together effectively. Techniques for this corneal laceration include using multiple simple interrupted sutures or a central purse-string suture with simple interrupted sutures added to close the linear portions.³³
Cornea and limbus/sclera laceration
In cases of a laceration involving both cornea and sclera, the limbus should be first approximated and sutured with 9-0 nylon, followed by repair of the affected cornea with 10-0 nylon, and lastly, the sclera is closed with 8-0 spatulated nylon suture. Any laceration extending beyond the limbus needs to be explored. If the laceration tracks beneath a rectus muscle (Figures 6 and 7), it may be necessary to hook and disinsert the muscles, perform the scleral repair, and then reposition the muscles. All lacerations should be closed as completely as possible. Very posterior lacerations can heal by fibrosis over 7–10 days.³⁴
Figure 6. Scleral laceration with uveal prolapse visible after a 360-degree conjunctival peritomy and hemostasis control. 7-0 silk sutures are used to hook the superior and lateral recti for better exposure.

Figure 7. Scleral laceration extending from the superior to lateral recti closed with multiple interrupted 8-0 vicryl sutures after excision of extruded uveal tissue. Conjunctival peritomy is then closed using 8-0 vicryl sutures.

With or without iris prolapse/damage
The prolapsed iris must be examined carefully. If the iris has been incarcerated for more than 24 hours, strong consideration should be given to excising it to reduce the risk of endophthalmitis. Moreover, if the iris appears nonviable, any portion of the iris that is outside the wound should be excised. Miotics such as acetylcholine chloride intraocular solution or carbachol intraocular solution 0.01% and viscoelastics can facilitate iris repositioning.³⁴ Any iris dialysis or irregularity that remains can be left alone at the time of primary corneal closure. Iridoplasty (using an artificial iris implant) can be performed later once the globe is stable.
Other considerations
If there is any violation of the anterior lens capsule with leakage of lens material, it is better to delay any lens-based surgery if possible and to manage inflammation and IOP medically. Small amounts of vitreous prolapse can involve cellulose amputation of strands and the use of viscoelastics to keep the vitreous back while the corneal wound closure is performed. If there are significant amounts of vitreous loss, then anterior vitrectomy may be required during the primary corneal trauma repair. A hyphema, if present, can be irrigated out through the corneal wound, and patients should be advised to sleep with their head of bed up, and IOP should be kept below 25 mmHg to avoid blood staining.³⁵⁻³⁶
Postsurgical Care and Follow-up
After the acute anterior segment repair is completed, refer the patient to a retina specialist to assess for retinal detachment and vitreous hemorrhage. Medical management typically includes oral and topical antibiotics, topical steroids, and IOP-lowering medications if needed. Monitor the patient closely over the first few weeks to diagnose any early signs of infectious endophthalmitis. The theoretical risk of sympathetic ophthalmia should be discussed with the patient if there was any iris prolapse with the original injury.³⁷ Finally, patients should be advised to wear polycarbonate glasses at all times to protect their remaining good eye.
Conclusions
Having a good management protocol and basic suturing competency can reduce anxiety regarding the primary surgical repair of emergency cases involving acute anterior segment trauma. Ophthalmologists can maintain proficiency in suturing skills by attending wet labs at conferences or placing the occasional suture in a controlled environment such as closure of a corneal wound in cataract surgery cases. Primary closure of globe ruptures should be in the skill set of every comprehensive ophthalmologist who can perform cataract surgery and take on-call responsibilities.
References
-
Nanji K, Gulamhusein H, Jindani Y, et al. Profile of eye-related emergency department visits in Ontario – a Canadian perspective. BMC Ophthalmol. 2023;23(1):305.
-
Chen A, McGwin G Jr, Justin GA, et al. The United States Eye Injury Registry: past and future directions. Ophthalmology. 2021;128(5):647-648.
-
Ahmed Y, Schimel AM, Pathengay A, Colyer MH, Flynn HW Jr. Endophthalmitis following open-globe injuries. Eye (Lond). 2011;26(2):212-217.
-
Haripriya A, Chang DF, Ravindran RD. Endophthalmitis reduction with intracameral moxifloxacin in eyes with and without surgical complications: results from two-million consecutive cataract surgeries. J Cataract Refract Surg. 2019;45(9):1226-1233.
-
Ng AL, Tang WW, Li PS et al. Intracameral cefuroxime in the prevention of postoperative endophthalmitis: an experience from Hong Kong. Graefes Arch Clin Exp Ophthalmol. 2016;254(10):1987-1992.
-
Endophthalmitis Study Group, European Society of Cataract & Refractive Surgeons. Prophylaxis of postoperative endophthalmitis following cataract surgery: results of the ESCRS multicenter study and identification of risk factors. J Cataract Refract Surg. 2007;33(6):978-988.
-
Erickson BP, Feng PW, Liao SD, et al. Dog bite injuries of the eye and ocular adnexa. Orbit. 2019;38(1):43-50.
-
McMaster D, Bapty J, Bush L, et al. Early versus delayed timing of primary repair after open-globe injury: a systematic review and meta-analysis. Ophthalmology. 2024 doi: 10.1016/j.ophtha.2024.08.030 [online ahead of print]
-
Gupta S, Mehta A. Open eye injury with full stomach. Internet Journal of Anesthesiology. 2013;22(2). Available at: https://ispub.com/IJA/22/2/6110.
-
Rho JE, Fowler BT, Armstrong GW, et al. Anesthesia for ruptured globe repair. EyeWiki. September 29, 2024. Available at: https://eyewiki.org/Anesthesia_for_Ruptured_Globe_Repair#cite_note-gupta8-8.
-
Pucchio A, Pur DR, Dhawan A, et al. Anesthesia for ophthalmic surgery: an educational review. Int Ophthalmol. 2023;43(5):1761-1769.
-
Bojikian KD, Stein AL, Slabaugh MA, et al. Incidence and risk factors for traumatic intraocular pressure elevation and traumatic glaucoma after open-globe injury. Eye (Lond). 2015;29(12):1579-1584.
-
Kuhn F, Morris R, Witherspoon CD, et al. The Birmingham Eye Trauma Terminology system (BETT). J Fr Ophtalmol. 2004;27(2):206-210.
-
Kate A, Sharma S, Yathish S, et al. Demographic profile and clinical characteristics of patients presenting with acute ocular burns. Indian J Ophthalmol. 2023;71(7):2694-2703.
-
Clare G, Bunce C, Tuft S. Amniotic membrane transplantation for acute ocular burns. Cochrane Database Syst Rev. 2022;9(9):CD009379.
-
Singla E, Jha UP, Muralidharan S, et al. Management of multi-surface ocular burns caused by molten iron. Trauma Case Rep 2023;48:100925.
-
Logothetis HD, Leikin SM, Patrianakos T. Management of anterior segment trauma. Dis Mon. 2014;60(6):247-253.
-
Lim CH, Turner A, Lim BX. Patching for corneal abrasion. Cochrane Database Syst Rev. 2016;7(7):CD004764.
-
Sharma N, Sah R, Priyadarshini K, et al. Contact lenses for the treatment of ocular surface diseases. Indian J Ophthalmol. 2023;71(4):1135-1141.
-
Algarni AM, Guyatt GH, Turner A, et al. Antibiotic prophylaxis for corneal abrasion. Cochrane Database Syst Rev. 2022;5(5):CD014617.
-
Shadmani A, Wu AY. Navigating the path to corneal healing success and challenges: a comprehensive overview. Eye (Lond) 2025 doi: 10.1038/s41433-025-03619-2 [online ahead of print]
-
Miller DD, Wagner IV, Ten Hulzen RD, et al. Delayed corneal healing after the use of topical ophthalmic anesthetics. Cureus. 2024;16(9):e70455.
-
Dang DH, Riaz KM, Karamichos D. Treatment of non-Infectious corneal injury: review of diagnostic agents, therapeutic medications, and future targets. Drugs. 2022;82(2):145-167.
-
Xu K, Kam KW, Young AL, et al. Recurrent corneal erosion syndrome. Asia Pac J Ophthalmol (Phila). 2012;1(6):349-354.
-
Solomon KD, Fernandez de Castro LE, Sandoval HP, et al. LASIK world literature review: quality of life and patient satisfaction. Ophthalmology 2009;116(4):691-701.
-
Legault GL, Kumar B. Corneal Laceration Repair. [Updated 2023 Jul 3]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan. Available from: https://www.ncbi.nlm.nih.gov/books/NBK576444/
-
Romanchuk KG. Seidel’s test using 10% fluorescein. Can J Ophthalmol. 1979;14(4):253-256.
-
Hersh PS, Zagelbaum BM, Kenyon KR, Shingleton BJ. Surgical management of anterior segment trauma. July 11, 2016. Available at: https://entokey.com/surgical-management-of-anterior-segment-trauma/.
-
Huang L, Larson J. Nonsurgical management of a traumatic, full-thickness corneal laceration: a case report. WMJ. 2024;123(4):307-310.
-
Lim CH, Turner A, Lim BX. Patching for corneal abrasion. Cochrane Database Syst Rev 2016;7(7):CD004764.
-
Lin DT, Webster RG Jr, Abbott RL. Repair of corneal lacerations and perforations. Int Ophthalmol Clin. 1988;28(1):69-75.
-
Hamill MB. Corneal and scleral trauma. Ophthalmol Clin N Am. 2002; 15(2):185-194.
-
Brooks SE, Johnson A, Kreuger EA. Novel technique to close stellate corneal lacerations. Cornea. 2024 Oct 29. doi: 10.1097/ICO.0000000000003730. Online ahead of print.
-
Johnson AJ, Townley R III, Pasternak JF. Damage control surgery: Blast – anterior segment trauma. In: Ophthalmology in Military and Civilian Casualty Care. Cham (Switzerland); Springer Nature; 2019.
-
Read J. Traumatic hyphema: surgical vs medical management. Ann Ophthalmol. 1975;7(5):659-662, 664-666, 668-670.
-
Little BC, Aylward GW. The medical management of traumatic hyphaema: a survey of opinion among ophthalmologists in the UK. J R Soc Med. 1993;86(8):458-459.
-
Parchand S, Agrawal D, Ayyadurai N, et al. Sympathetic ophthalmia: A comprehensive update. Indian J Ophthalmol. 2022;70(6):1931-1944.
Dr. Huertas-Bello is a cornea, external diseases, and refractive surgery fellow, Department of Ophthalmology & Vision Sciences, University of Toronto, and a Refractive Surgeon, TLC Laser Eye Centres, Toronto, Ontario.
Dr. Bareket is a cornea, external diseases, and refractive surgery fellow, Department of Ophthalmology & Vision Sciences, University of Toronto, Toronto, Ontario.
Dr. Chan is an Associate Professor, Department of Ophthalmology & Vision Sciences, University of Toronto, and Medical Director, The Eye Bank of Canada – Ontario Division. She is also a LASIK Surgeon at TLC Yonge Eglinton and is affiliated with the Kensington Eye Institute, Toronto Western Hospital, and St. Michael’s Hospital, Toronto, Ontario.
Financial Disclosures
Drs. Huertas-Bello and Bareket stated that they have no disclosures to report in association with the contents of this issue. Dr. Chan has received prior honoraria for consulting services from AbbVie, Admare Bioinnovations, Aurion Biotech, Bausch & Lomb, Claris Bio, Vital Tears, Johnson & Johnson, Kala Ophthalmics, Labtician Ophthalmics, Sun Ophthalmics, Thea, and Valeo Pharma. She has also received research grant support from Aurion Biotech, Corneat, Claris Bio, and Design Therapeutics.
Ophthalmology Rounds is made possible through educational funding
from the following industry co-sponsors:
Supporters: Apobiologix, Astellas, Biogen, Roche
Friends: Alcon, Apellis, Bayer